Case Study: XprESS
What is XprESS?
XprESS is one of the technologies listed under the MedTech Funding Mandate policy, which aims to accelerate equitable patient access to The National Institute for Health and Care Excellence (NICE) recommended devices, diagnostics, or digital products. These innovations aim to deliver tangible benefits to the NHS and its patients, are cost-saving within 3 years, and are affordable for the NHS to implement.
XprESS balloon dilation is a minimally invasive device to treat chronic sinusitis patients without any nasal polyps. Routine rhinology surgery known as functional endoscopic sinus surgery (FESS) requires general anaesthetic (GA) and an operation theatre to perform the procedure. XprESS is a minimally invasive procedure which can be performed under local anaesthetic (LA) and doesn’t require an operation theatre as it can be performed in a suitable treatment room. XprESS can therefore contribute to reducing elective care waiting lists in line with current NHS priorities and operational planning guidance.
What were the aims?
This project aims to support the spread and adoption of the XprESS balloon dilation device to healthcare providers to accelerate equitable access to patients within the West Midlands region. XprESS aims to reduce the waiting times and the number of patient journeys to the hospital for treatment, as well as reduce the need for more invasive procedures compared to FESS to help lower the risk of some complications, less nasal bleeding, quick patient recovery and improved quality of life.
As we progress into the second year of XprESS being on the MTFM, we will use information gathered during the 1st year including baseline uptake in the West Midlands, interest in implementation and availability of funding for implementation to determine target levels of adoption.
What did we do?
HIWM is working closely with the supplier, Stryker, and has shared responsibilities to support the implementation and adoption of the technology in the 12 Trusts in the region with services that could utilise the technology for sinus surgery in line with NICE MTG30. Given the variability of eligible providers, we have to be versatile and employ elements that occur in several theoretical frameworks of innovation adoption.
To create a case for change, we assembled a core offer of materials to support engagement with providers and prompt decisions to adopt XprESS. These materials shared qualitative information regarding prospective clinical and operational benefits to implementation of XprESS. Quantitative information was also shared regarding potential cost benefits derived from trust-level procedure data obtained from NHS RightCare colleagues. These also took into account implementation costs specific to the scenario (e.g. use under general anaesthetic or local anaesthetic) to ascertain the likelihood of in-year savings. Collectively these materials were designed to help XprESS demonstrate several characteristics associated with positive decision-making around adoption such as cost-efficacy, clear evidence, compatibility with local practice, low risk and ease of use/trialling. This information was supplemented by detailing the support providers could expect from the HIWM throughout implementation.
Understanding the importance of networking to innovating at an inter-organisational and inter-system level and the importance of strong local leadership/innovation champions, we have sought to engage and coordinate local personnel around each Trust’s implementation to contain the expertise, influence and enthusiasm to facilitate progress. Establishing initial engagement required persistence and different initial contacts from which a network was formed. Between providers, these networks have commonalities like direct clinical involvement (e.g. ENT Consultants), operational management (senior e.g. Director of Operations/Associate Divisional Director and/or more localised managers e.g. Directorate/Care Group Manager) and finance/commissioner involvement. Additional facilitators form part of these variable networks (e.g. Heads of Innovation, theatre staff), which will likely further expand as implementation and business cases progress (e.g. procurement).
Among our additional early project activities have included ensuring the tools to which we might direct our local stakeholders are up-to-date and user-friendly. We have therefore worked with NICE to modify fields on the XprESS NICE resource impact template to help Trusts in the region easily work out estimated cost/ savings benefits before adopting this technology. We are additionally ensuring we are able to share best practices by engaging with prevalent existing users of XprESS within the West Midlands, as well as meeting monthly with national leads, our East Midlands Health Innovation Network colleagues, to share known barriers and facilitators of implementation.
What were the outcomes?
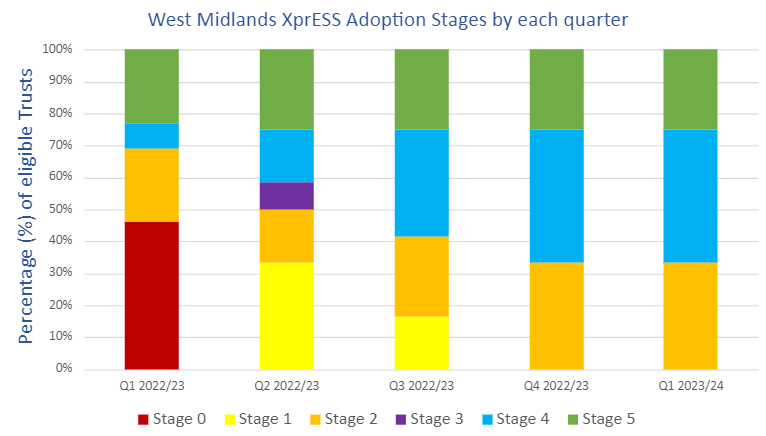
Within the West Midlands region, there are 12 Trusts with services that could adopt XprESS for use according to NICE MTG30. We have 8 Trusts (67%) either implementing or adopting XprESS as of Q4, up 31% from our baseline Q1 figures. Of these 8 Trusts, three (25%) have adopted and are using XprESS for their patients with a further five Trusts (42%) at the implementation stage.
From baseline levels of uptake and implementation progress, we have collectively facilitated progress through 17 implementation stages since Q1, among which 2 of our 5 implementing Trusts had no prior knowledge of XprESS during Q1 and have progressed 4 stages each respectively.
Key:
Stage 0 = No information
Stage 1 = Knowledge of XprESS
Stage 2 = Interest in XprESS
Stage 3 = A decision to not adopt XprESS
Stage 4 = Implementing XprESS
Stage 5 = Adopted XprESS
As part of this progress, we have now engaged with all Trusts who could utilise XprESS to treat chronic sinusitis. Furthermore, we have built small networks around implementation and strong relationships with ENT consultants from several Trusts who are keen to support their peers regionally and nationally to adopt this innovation by sharing their knowledge and experience.
Taking figures of FESS procedures in the West Midlands from April 2021 – March 2022 as a guide and NICE estimated cost savings of £152 per patient, if XprESS were to be used optimally (i.e. 80% of cases) for FESS in the West Midlands, cost savings from this technology could total £50,464 per year across our region. Our early progress is helping us work towards this total, as well as realising benefits to patient waiting times and removing elective care backlogs.
“The balloon sinuplasty with XprESS is a part of the new service model of ‘Awake Rhinology’ which has brought many opportunities and benefits. These include maximising throughput of General Anaesthetic cases in theatres by shifting appropriate Local Anaesthetic cases out of main theatres, which means fewer steps in the patient journey, shorter waiting times for patients and reduction in the overall backlog of chronic sinusitis patients since the pandemic which has resulted in cost savings and improved services.” – Mr James Barraclough, Consultant Rhinologist, Facial Plastics and ENT Surgeon at Royal Wolverhampton Hospital.
What are the next steps?
Our ongoing activities to further progress local implementation and adoption of XprESS as our project progresses further will include:
- Continued prioritisation of implementation in sites likely to experience the greatest benefits of using XprESS and continued communication of Trust-specific benefits to facilitate decisions on implementation.
- Expanding local networks to increase the involvement of influential decision-making individuals and boards within providers and ICBs, as well as increased commissioner involvement.
- Active contributions to business cases as these progress during the second year
- Identification and sharing (including peer sharing) of best practices around the use of XprESS, particularly under local anaesthetic.
- Identification and articulation of additional policy and strategic levers to facilitate engagement and decisions on implementation
- If acceptable to the supplier, monitor purchase data to enable quantification (and/or estimation) of potential clinical impacts and cost savings.
For those who would like to find out more about uptake of XprESS in the West Midlands, who are interested in implementing XprESS or who would like to know more about best practices relating to use of XprESS, please email Kanwal.Umar@healthinnovationwm.org.
